Abstract: The gas chromatography method for measuring the water content of interior wall coatings is introduced, and the accuracy and precision of the method are investigated. The method is simple and feasible, accurate and reliable, and is suitable for various interior wall paint samples.
Keywords: gas chromatography; internal standard method; water content
CLC number: TQ630.7 * 2 Document identification code: A Article number: 0253-4312 (2003) 11-0048-03
0 Preface
Since the promulgation of the national compulsory standard GB18582-2001, in order to meet the VOC index requirements of its harmful substances, it is necessary to accurately measure the moisture content of interior wall coatings. The water content of the interior wall paint is about 40%. If the water content is measured by Karl Fischer method, it is said that the sample volume is difficult to control, and the Karl Fischer reagent is drawn many times, and several readings will cause titration errors; The film-forming substance in the coating is a polymer emulsion. During the titration process, the emulsion breaks continuously, releasing water molecules, which will also bring about titration errors. In order to solve this problem, we use a small-bore capillary weak polarity column, and use a thermal conductivity detector (TCD) to detect the temperature rise of the program. The separation effect is very ideal, and the recovery rate of the standard addition is> 99%. The degrees are in accordance with the requirements of GB18582-2001. The method has strong operability and is suitable for various interior wall paint samples.
1 Experimental part
1.1 Instruments and reagents
Apparatus: electronic analytical balance (one hundred thousandth), GC9160 gas chromatograph (need to be equipped with splitter), 1.0μL micro sampler, 10mL vials with rubber stopper, medical glass syringes (2mL, 5mL each) ), A 2mL pipette, a small-bore capillary column ECTM-5 (30m × 0.32mm × 1.0μL), integrator or chromatography workstation.
Reagents: water (purified water or double distilled water); dimethylformamide (chromatographically pure); internal standard: absolute ethanol (chromatographically pure).
Before using ethanol and dimethylformamide, the Karl Fischer method should be used to determine its water content. If the water content is greater than 0.01%, it must be dried and dehydrated until it meets the requirements.
1.2 Measuring principle
Add an appropriate amount of internal standard to the uniformly mixed sample, dilute with a little dimethylformamide, shake well, take the supernatant (0.3μL) and inject it into the gas chromatograph, the sample is carried into the chromatography by the carrier gas (H2) In the chromatographic column, separate the water from other volatiles in the sample, use a thermal conductivity cell detector and record the chromatogram, and use the internal standard method to calculate the water content of the sample.
1.3 Measurement conditions
Vaporization temperature: 200 ℃; Reference gas: 35kPa; Auxiliary hydrogen: 60kPa; Detection temperature: 180 ℃; Bridge flow: 150mA; Carrier gas: hydrogen, purity ≥99.99%, silica gel + 5A molecular sieve to remove water and oil, in front of the column Pressure 60kPa; Program temperature increase: initial temperature 65 ℃, hold for 12min, heating rate 50 ℃ / min to 200 ℃, hold for 3min; split ratio: 50: 1; injection volume: 0.3μL.
1.4 Determination of the relative quality correction factor
1.4.1 Preparation of standard samples
Weigh 1.15g of purified water (accurate to 0.0002g) in a 10mL sample bottle and 0.2g of chromatographic pure absolute ethanol (accurate to 0.0002g) to make the peak area ratio of the sample and the internal standard close to 1. ), Add 2mL of anhydrous dimethylformamide as a diluent with a 2mL pipette or glass syringe, seal and shake well (Note: quickly close the sample bottle after each weighing to prevent the sample from volatile loss).
1.4.2 Determination of the relative quality correction factor
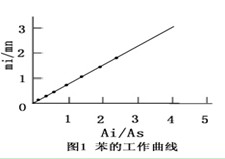
After the instrument is stable, use a 1.0μL micro sampler to draw 0.3μL of standard sample into the gas chromatograph, and record the chromatogram and chromatographic data. The chromatograph runs the program once according to the analysis conditions, and can continuously inject 4 to 5 needles of the standard sample (because the peak of water and internal standard comes out within 2 minutes, and the solvent dimethylformamide peaks only after about 12 minutes, it can be continuous Inject). The chromatogram of a typical interior paint is shown in Figure 1.
Figure 1 Chromatogram of typical interior wall paint
1.4.3 Calculation of relative quality correction factor
The formula for calculating the mass correction factor Fi for pure water versus absolute ethanol is as follows:
Where: mi-the quality of pure water, g;
ms——the quality of the internal standard substance absolute ethanol, g;
Ai-Peak area of ​​pure water;
AS-the peak area of ​​the internal standard ethanol.
If ethanol and dimethylformamide are not anhydrous reagents, then use the same amount of ethanol and dimethylformamide mixed solution (without adding water) as a blank, accurately take 0.3μL of this blank solution into the gas chromatograph, and record the blank water The area of ​​the peak can be fed into the needle 5 times in succession, and the average value is taken as the peak area B of the blank water. Calculate the relative correction factor Fi of water as follows:
In the formula: A——peak area of ​​water;
Fi-the relative quality correction factor of water;
B——Peak area of ​​blank water;
The meanings of ms, mi and As are the same as above.
Continuous parallel measurement of the parallel deviation of the relative mass correction factor Fi of the water to the internal standard ethanol should be less than 0.05.
1.5 Linear range of the method
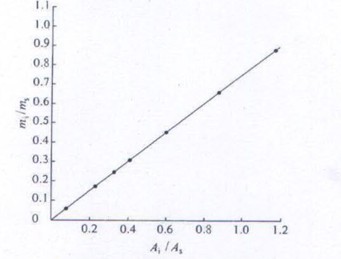
Prepare a series of standard solutions according to the above determination method and analyze according to the above chromatographic conditions, using Ai / As as the abscissa and mi / ms as the ordinate to make the working curve, as shown in Figure 2. The data is shown in Table 1.
Figure 2 Working curve of water relative to the internal standard ethanol
Table 1 Linear range of water relative to internal standard ethanol
Water quality / g
Ai / As
mi / ms
Internal standard (ethanol)
0.0106
0.07258
0.05375
0.1972
0.0330
0.2276
0.1643
0.2008
0.0478
0.3338
0.2398
0.1993
0.0640
0.4140
0.3108
0.2059
0.0963
0.6072
0.4492
0.2144
0.1324
0.8659
0.6594
0.2008
0.1914
1.1696
0.8712
0.2197
0.2061
2.0186
1.002423
0.2056
1.6 Standard recovery test
In order to prove the reliability of the measurement results, weigh a certain amount of purified water, add 2mL of dimethylformamide, prepare several sets of solutions of known concentration, measure the water content of each solution according to the above analysis method, and calculate the recovery rate. Table 2.
Table 2 Test results of standard recovery
Theoretical value of water in the preparation solution /%
Actual measured value of water /%
Standard recovery rate /%
30.23
30.45
100.73
28.30
28.96
102.33
1.7 Determination of samples
After stirring the sample evenly, accurately weigh 0.4-0.6g (accurate to 0.0002g) sample and 0.2g (accurate to 0.0002g) internal standard in a 10mL sample bottle, add 2mL of dimethylformamide with a pipette, Seal, shake vigorously for 10 minutes, and let stand for 5 minutes to precipitate. Aspirate 0.3μL of the supernatant in the sample bottle with a 1.0μL micro sampler, measure the sample under the same chromatographic conditions as the correction factor, record the chromatogram and chromatogram data of each component on the chromatographic column Qualitative relative to the retention time of the internal standard.
1.8 Calculation of results
The mass fraction Xi (%) of the water in the sample is calculated as follows:
In the formula: Fi——the quality correction factor of water relative to the internal standard;
ms-the quality of the internal standard, g;
Ai——The peak area of ​​water in the sample;
mi-the quality of the sample, g;
As-the peak area of ​​the internal standard.
Take the arithmetic mean of the results of four parallel measurements as the measurement result of the sample water content.
1.9 Reproducibility
The parallel deviation of the results of two measurements by the same operator should not exceed 1.0%.
1.10 Precision of measurement results
The precision data of the measurement results are shown in Table 3.
Table 3 Precision of measurement results
Weighing sample / g
The measurement results/%
average value/%
Relative deviation/%
standard deviation/%
0.6758
40.36
40.73
-0.0092
0.3159
0.5984
40.70
-0.00074
0.4727
40.93
0.0049
0.3815
40.91
0.0044
It can be seen from Table 3 that the precision of this method is very high, and its accuracy is very ideal.
2 Results and discussion
(1) The analytical method uses a weak polar small-bore capillary column, the injection volume should not be greater than 0.3 μL, and the split ratio must be large enough, otherwise the separation effect is poor, the symmetry of the peak row is also poor, and the capillary column has been damaged.
(2) The vaporization chamber is equipped with a glass liner, filled with an appropriate amount of treated glass wool to filter the residue, and according to the frequency of sample preparation, it is appropriate to change the liner once every 2 days.
(3) Run the program once to increase the temperature, and the needle can be inserted 5 times, that is, the second needle can be quickly entered after the internal standard ethanol is peaked. Each sample must be run once the program is completed, because the solvent dimethylformamide has a higher boiling point, the peak time under the above analysis conditions is 11 ~ 12min.
(4) If the laboratory is not equipped with a capillary column or no shunt device, a 2m × 0.3mm stainless steel packed column can also be used, filled with domestic GDX-102, 80/100 mesh packing.
(5) If the reagent used is not an anhydrous reagent, that is, when the water content measured by Karl Fischer reagent is> 0.01%, a blank test must be performed and the blank water peak area must be subtracted, and each time a sample is taken, The injection volume of standard samples, samples and blanks should be strictly controlled and consistent (accurately controlled at 0.3 μL), and accurately add 2.0 mL of dimethylformamide diluent with a pipette.
Stencil Brush
Types of brushes.There are so many many brushes:washes/glazes, rounds, flats, filberts and liners and more. Choosing the right type for the technique is important. Let`s look at the different types.Flat Brushes-Flats are brushes with a straight chisel edge and square shaped filaments. These can be known as shades if they`re in smaller sizes and washes/glazes if in bigger sizes. Large areas are painted with a wash/glaze brush whereas smaller flats are used for small areas of painting. Round brushes-Round brushes have a large diameter of the ferrule, more so than a liner, which can be used for applying thick to thin lines, filling in odd shaped areas, painting details and work great for lettering. Liners don`t hold as much paint as a round, however, they are super great for creating lines or curves. Script liners are similar to liners but the filaments are much longer and hold more paint. A round brush tapers to a pointed tip – several types of rounds are referred to rounds, liners or script liners. Angle brushes are filaments that have been cut on an angle – these are excellent to use in small or curved areas of the painting.Filberts Brushes with oval shaped filaments are known as filberts in smaller sizes and oval wash in larger sizes. Both shapes can be used for base coating, stroke work and more. There are filbert combs, filbert wash and more.Mop Brushes,Then there are mop brushes–great for blending and smoothing out small areas. They`re also ideal for applying powdered pigments–just dust them lightly over hot or tacky wax.Specialty Brushes-Then there are all the speciality brushes on the market used for certain techniques and garner their own results–fan, deer-foot, stumbler, mops,stencil and more.
Stencil Brush for Painting, Paint Brush, Boar Painting Brushes
SAMINA FORAM (SHENZHEN) CO., LIMITED. , https://www.saminamakeuptools.com