Swine Epidemic Diarrhea Virus Antibody (PEDV) Enzyme-Linked Immunosorbent Assay (ELISA) Kit Instructions for Use This kit is for research use only. This kit is for the determination of epidemic diarrhea virus antibodies (PEDV) in swine serum, plasma and related fluid samples. )Level. Experimental principle: This kit uses double antigen sandwich method to determine porcine epidemic diarrhea virus antibody (PEDV) in serum or plasma. The microporous plate is coated with purified porcine epidemic diarrhea virus antibody (PEDV) antigen to prepare a solid phase antigen, which can be combined with the epidemic diarrhea virus antibody (PEDV) in the sample to wash away unbound antibody and other components. It is then combined with the HRP-labeled epidemic diarrhea virus antibody (PEDV) antigen, and after thorough washing, the substrate TMB is added for color development. TMB is converted to blue under the catalysis of HRP enzyme and converted to the final yellow color by the action of an acid. The absorbance (OD value) was measured at a wavelength of 450 nm using a microplate reader, and compared with the CUTOFF value to determine the presence or absence of a porcine epidemic diarrhea virus antibody (PEDV) in the specimen. Kit composition: Kit composition 48-well configuration 96-well configuration Storage instructions 1 part 1 part sealing film 2 pieces (48) 2 pieces (96) Sealed bag 1 1 enzyme label coated plate 1 × 48 1 × 96 2 -8 °C preservation negative control 0.5ml × 1 bottle 0.5ml × 1 bottle 2-8 ° C Preservation positive control 0.5ml × 1 bottle 0.5ml × 1 bottle 2-8 ° C Preservation enzyme standard reagent 3 ml × 1 bottle 6 ml × 1 Bottle 2-8 °C Preservation Sample Diluent 3 ml × 1 bottle 6 ml × 1 bottle 2-8 ° C Preservation developer A solution 3 ml × 1 bottle 6 ml × 1 bottle 2-8 ° C Preservation developer B solution 3 Ml × 1 bottle 6 ml × 1 bottle 2-8 ° C Preservation solution 3 ml × 1 bottle 6 ml × 1 bottle 2-8 ° C Preservation concentrated washing solution (20ml × 20 times) × 1 bottle (20ml × 30 times) × 1 bottle 2-8 ° C preservation sample processing and requirements: 1. Serum: room temperature blood solidification for 10-20 minutes, centrifugation for about 20 minutes (2000-3000 rev / min). The supernatant is carefully collected, and if precipitation occurs during storage, it should be centrifuged again. 2. Plasma: EDTA or sodium citrate should be selected as an anticoagulant according to the requirements of the specimen. After mixing for 10-20 minutes, centrifuge for about 20 minutes (2000-3000 rpm). The supernatant is carefully collected, and if a precipitate forms during storage, it should be centrifuged again. 3. Urine: Collect with a sterile tube and centrifuge for about 20 minutes (2000-3000 rpm). The supernatant is carefully collected, and if a precipitate forms during storage, it should be centrifuged again. The chest and ascites and cerebrospinal fluid are referred to. 4. Cell culture supernatant: When detecting secreted components, collect them in a sterile tube. Centrifuge for about 20 minutes (2000-3000 rpm). Collect the supernatant carefully. When the components in the cells were detected, the cell suspension was diluted with PBS (pH 7.2-7.4), and the cell concentration reached about 1 million/ml. By repeated freezing and thawing, the cells are destroyed and the intracellular components are released. Centrifuge for about 20 minutes (2000-3000 rpm). Collect the supernatant carefully. If a precipitate forms during storage, it should be centrifuged again. 5. Tissue specimen: After cutting the specimen, weigh the weight. Add a certain amount of PBS, pH 7.4. It was quickly frozen and stored in liquid nitrogen for use. The specimen still maintains a temperature of 2-8 ° C after melting. A certain amount of PBS (pH 7.4) was added, and the specimen was homogenized by hand or homogenizer. Centrifuge for about 20 minutes (2000-3000 rpm). Collect the supernatant carefully. One part of the package is to be tested, and the rest is frozen for use. 6. The specimens should be extracted as soon as possible after collection, and the extraction should be carried out according to the relevant literature. The experiment should be carried out as soon as possible after extraction. If the test cannot be performed immediately, the specimen can be stored at -20 °C, but repeated freezing and thawing should be avoided. 7. Samples containing NaN3 cannot be detected because NaN3 inhibits horseradish peroxidase (HRP) activity. Operation steps: 1. No.: The corresponding micropores of the sample are numbered sequentially. Each plate should be set with 2 holes of negative control, 2 wells of positive control and 1 well of blank control (the blank control well is not added with sample and enzyme standard reagent, and the other steps are operated. The same) 2. Loading: A negative control and a positive control (standard) 100 μl were added to the negative and positive control wells, respectively. Then, 50 μl of the sample diluent is added to the sample well to be tested, and then 50 μl of the sample to be tested is added. Mix gently by shaking, 3. Incubate: Cover with a sealing plate and incubate at 37 °C for 30 minutes. 4. Solution: Add 30 (48 times of 20T) concentrated washing solution to distilled water to 600ml and then use it. 5. Wash: carefully remove the sealing film, discard the liquid, dry, fill each well with washing liquid, and let stand. Discard after 30 seconds, repeat 5 times, and pat dry. 6. Add enzyme: add 50μl (or one drop) of enzyme labeling reagent to each well, except for blank wells. 7. Incubation: Operation is the same as 3. 8. Washing: operation is the same as 5. 9. Color development: Add 50 μl (or one drop) of developer A to each well, then add 50 μl (or one drop) of developer B, gently Shake and mix, color at 37 ° C for 15 minutes. 10. Termination: Add 50 μl (or one drop) of stop solution to each well to stop the reaction (the blue color turns yellow). 11. Measurement: The absorbance (OD value) of each well was measured sequentially with a blank air conditioner of zero and a wavelength of 450 nm. The measurement should be carried out within 15 minutes after the addition of the stop solution. Outcome judgment: Test validity: average value of positive control well ≥ 1.00; mean value of negative control ≤ 0.15 Critical value (CUT OFF) calculation: critical value = average value of negative control well + 0.10 (the average value of negative control is less than 0.05 and calculated by 0.05) Negative judgment: sample OD value
Related download
Porcine epidemic diarrhea virus antibody (PEDV) enzyme-linked immunosorbent assay (ELISA) kit instruction manual.rar
Click to enter the booth of Shanghai Yuanyan Biotechnology Co., Ltd. to see more I want to contribute


Sweep, welcome attention
Education Equipment Purchasing Network Official WeChat
Master the latest and most authoritative information in the education equipment industry
Copyright and Disclaimer:
1 All the works of the “Source: China Education Equipment Purchasing Network†are copyrighted by the China Education Equipment Purchasing Network. They may not be reproduced, extracted or used in other ways without the authorization of this website. Works that have been authorized by this website should be used within the scope of authorization, and indicate "Source: China Education Equipment Purchasing Network". Offenders will be held accountable for relevant legal responsibilities.
2 Any work that states "Source: XXX (not this website)" is reproduced from other media. The purpose of the reprint is to transmit more information. It does not mean that this website agrees with its views and is responsible for its authenticity. Direct responsibility and joint liability for the infringement of such works. If other media, websites or individuals download and use from this website, they must retain the "source of the manuscript" indicated on this website, and bear the legal responsibility for copyright.
3 If you are involved in the content of the work, copyright, etc., please contact the website within two weeks from the date of publication of the work, otherwise it will be deemed to have waived the relevant rights.
Manual Delivery Table
Manual Delivery Table
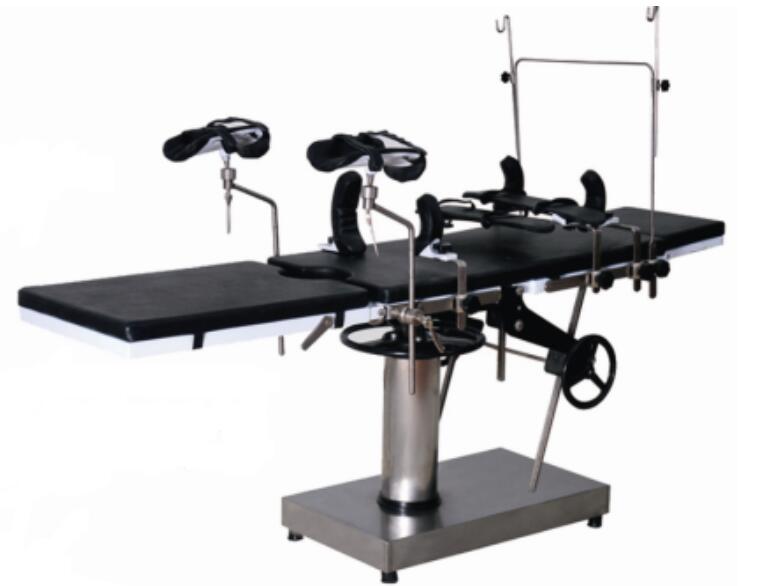
1. Import hydraulic system
2. Memory sponge mattress
3. Electric Longitudinal displacement ≥350mm
4. Tabletop is X-ray available
5. Optional Carbon fiber bed panel
This Manual Delivery Table is widely used in operating rooms of hospital and clinics. It is suitable for surgery operations on body part
like purposes head, neck, chest and abdominal cavity, perineum and extremities for surgery, obstetrics and gynecology, ENT, orthopedics
and other surgical operations.
The Manual Delivery Table can be flexibly adjusted by the foot pedal pump and manual hand wheels. Adjustments include waist bridge lift,
head board folding up and down, Backboard folding up and down, leg board folding down, tabletop lean left and right, tabletop lead forward
and backward .
Material of the whole structure is made of 304 stainless steel.

Certificates:
Certificates of CE, ISO9001, ISO13485, CFDA are approved.

Our company

Shangdong province is the main machinery production base in China.
KANGERJIAN Medical Technology Co., Ltd. is a group of senior lighting design expert and machinery manufacturing expert company with 20years experience and factory locating in the east city--the hometown of confucius--Qufu in Shandong province, China.
The Company has passed the ISO:9001:2008 quality system certification, ISO13485:2003 quality system certification, CE certification and CFDA certification, so that the enterprise management standards and product quality is relatively connected to expand the international market for enterprises to lay the foundation.
Our main products: Operation Theatre Lights, Halogen Operating Light, LED Operating Light , Double Dome Operating Lamp, Single Dome Operating Light, Surgical Table, Medical Electric Operating Table, Electric Hydraulic Operating Table, Obstetric Delivery Table, Gynecology Examination Tables, Economical Gynecology Table, Electrical Gynecological Table, Operating Theatre Pendants, medical hanging tower, ICU tower crane in ICU room, LED Viewbox etc. medical equipment.
The quality of casting by me, the market led by me! Excellent from professional, KANGERJIAN people lead the new trend of medical equipment.
KANGERJIAN Team

Package:
Inner Package---Dust-proof plastic film+ qualified shockproof foam
Outer Package---Thick Plywood case for over sea delivery
We support ODM of the package if there's special demands from clients
Shippment:
Sea shipping, Air transportation, Expresses like DHL, UPS FEDEX, TNT etc. Our forwarder give good price support.
Clients also can choose their own forwarder.

Manual Delivery Table,Obstetric Delivery Bed,Manual Gynaecology Delivery Table,Manual Multifunction Delivery Table
Shandong qufu healthyou Medical Technology co.,Ltd , https://www.kangerjianmedical.com









